Focus On: SARS-CoV-2
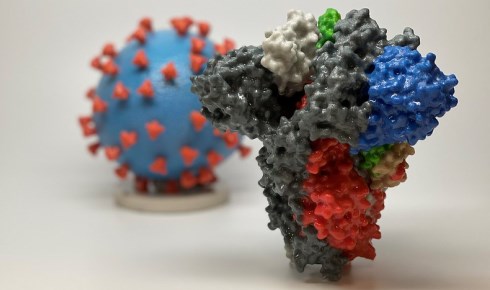
Above: A 3D print of a spike protein of SARS-CoV-2 and SARS-CoV-2 virus particle (courtesy NIH). The spike protein is the focus of efforts to find a vaccine.
The Biologist looks at the latest scientific information on the coronavirus SARS-CoV-2, which has spread rapidly around the world since December 2019.
Read the Biologist's new Q&A series on how biologists have sprung into action to fight SARS-CoV-2.
February 24th 2020
Public health experts, epidemiologists, immunologists and virologists around the globe have been working to understand, track and contain the coronavirus that emerged in the city of Wuhan in China last year. Research is focusing on the distinctive protein spikes of the virus and how the body fights the infection, informing the emergency development of point-of-care diagnostic tests, repurposed therapeutics and potential vaccines.
The virus
In February the International Committee on Taxonomy of Viruses named the new virus SARS-CoV-2 after determining that it formed a ‘sister clade’ to other coronaviruses of bats and humans, such as SARS-CoV (commonly known as SARS) of the established species Severe acute respiratory syndrome-related coronavirus.
Like other coronaviruses, SARS-CoV-2 particles are made up of a spherical envelope with spike-like proteins protruding from their surface - the name ‘coronavirus’ stems from the crown-like appearance of the spikes.
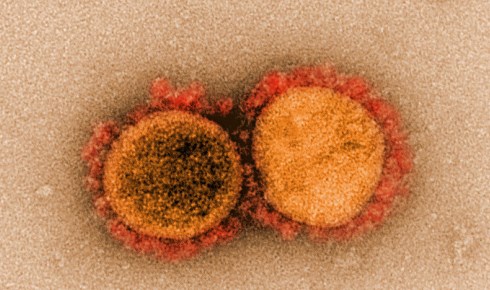 Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility in Fort Detrick, Maryland. As with SARS, the spikes of SARS-CoV-2 bind to a receptor on human cells called angiotensin-converting enzyme 2 (ACE2). Once bound, the spike proteins undergo a structural change that enables the viral membrane to fuse with the cell membrane and insert genetic material into the cell.
Studies of the SARS-CoV-2 spike found it could be up to 10 to 20 times more likely to bind to ACE2 on human cells than the spike from the SARS-CoV virus. This may enable SARS-CoV-2 to spread more easily from person to person than SARS, which caused around 8,000 cases of respiratory illness in 26 countries between 2002 and 2003.
Open-source databases such as www.nextstrain.org are helping to track the evolution of the virus as users upload hundreds of genetic sequences from different strains around the world.
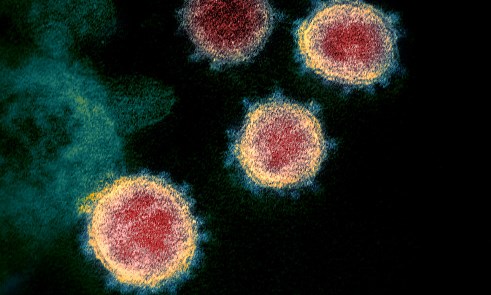 Above: A transmission electron microscope image showing SARS-CoV-2, isolated from a patient in the US, emerging from the surface of cells cultured in the lab. The 'corona' of spike proteins around the particle can be seen in blue. Courtesy of NIAID-RML (National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratories)
Above: A transmission electron microscope image showing SARS-CoV-2, isolated from a patient in the US, emerging from the surface of cells cultured in the lab. The 'corona' of spike proteins around the particle can be seen in blue. Courtesy of NIAID-RML (National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratories)COVID-19
In February the World Health Organisation (WHO) named the disease caused by the new virus COVID-19 (‘CO’ for corona, ‘VI’ for virus, ‘D’ for disease, 2019). People over 60, or those with pre-existing heart, lung or immune system conditions are particularly at risk of more severe infections leading to complications such as pneumonia and septic shock.
Although the general view is that most people outside these groups will suffer from only mild flu-like symptoms, data from the CDC shows that up to a fifth of infected people in the US aged 20-44 have been hospitalised, including 2%-4% who required treatment in an intensive care unit.
More recently, studies have linked higher viral load with more severe symptoms, suggesting that a those exposed to lots of viral particles (for example by prolonged contact with an infected person, or lots of infected persons) may be more likely to develop serious cases of COVID-19.
Testing and diagnosis
As of March, dozens of projects were underway to improve the way people are tested for COVID-19. Initial tests, which required samples of mucus or saliva to be sent to specialist laboratories, meant suspected cases could not be confirmed for up to 10 days. Many researchers also questioned the effectiveness of the CDC’s primer sequences for PCR assays and roll-out of testing kits.
Scientists from the University of Oxford and biotech firms such as Mologic and Assay Genie in Ireland have been developing rapid point-of-care diagnostics to detect the presence of SARS-CoV-2 in as little as 15-30 minutes. The government has also stressed the need for more sensitive tests that can detect the virus in those without symptoms.
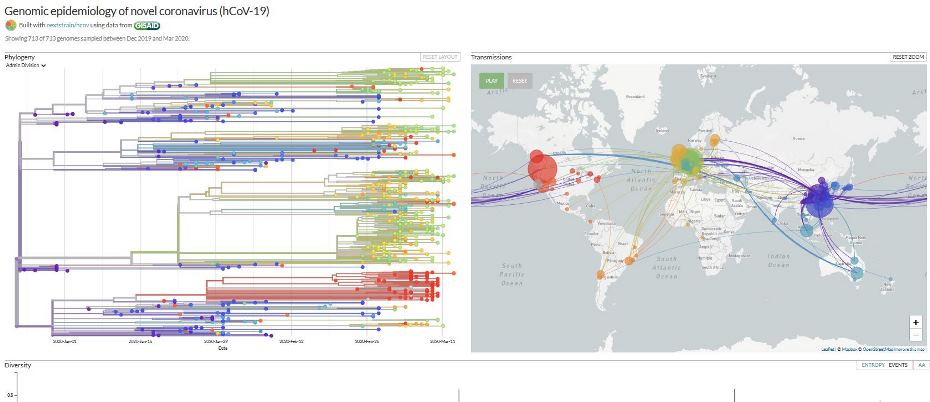 The website nextstrain.org is compiling real-time phylogenic maps tracking the evolution of strains using genomic sequence data from around the world.
The website nextstrain.org is compiling real-time phylogenic maps tracking the evolution of strains using genomic sequence data from around the world. Transmission
It is believed that the basic reproductive number of COVID-19 – i.e. the number of new cases generated by each infected person – is between 2.0 and 3.0, higher than flu (1.3). As with the estimates of mortality rate, data is highly variable depending on the testing regime and social policies in force in each region.
The main way the virus spreads was thought to be via water or mucus droplets passed from person to person. But research by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) found the virus is also stable in aerosols for up to three hours and for days on surfaces such as plastic and steel. Another study by researchers in China found that higher temperatures and humidity could be linked to slightly lower transmission rates.
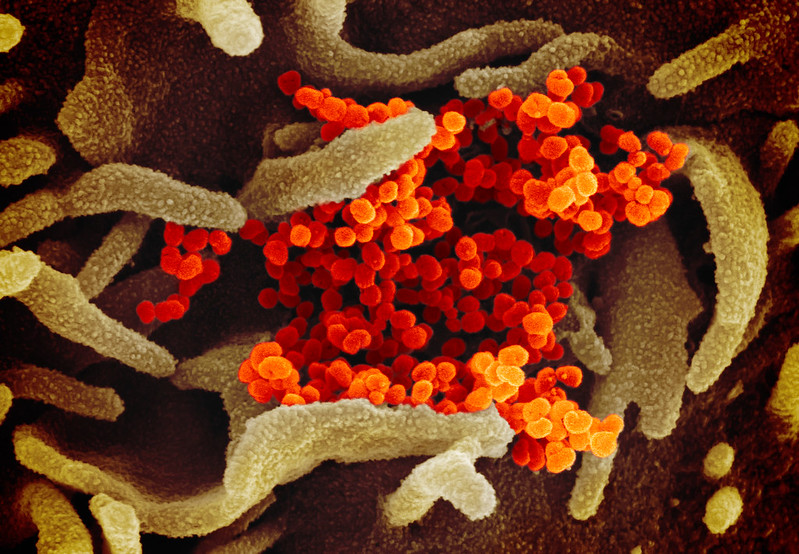 Another scanning electron microscope image showing SARS-CoV-2 emerging from the surface of cells cultured in the lab, courtesy of NIAID-RML
Another scanning electron microscope image showing SARS-CoV-2 emerging from the surface of cells cultured in the lab, courtesy of NIAID-RMLTherapeutics and a vaccine
Reports suggest that a drug used in Japan to treat new strains of influenza, known as favipiravir or avigen, could be effective in helping those with COVID-19 recover. Further international collaborations are testing existing drugs in the hope they can be repurposed to treat or prevent infections in some way.
Human trials for two vaccines began in March after the authorities agreed to fast track the drugs and allow animal studies to be conducted concurrently. One, an mRNA-based drug made by NIAID scientists and Moderna, encodes for the distinctive spike protein of SARS-CoV-2, allowing the body to create the protein in its cells and then recognise it as foreign. The other, made by Inovio, is based on viral DNA. Other vaccines in development are based on the protein spike itself or attenuated viral particles.
Despite the impressively rapid progress to human trials, drug development experts have said it is likely to take at least a year before any vaccine can be fully tested, manufactured and deployed. In the meantime, people are advised to practise good hand hygiene, avoid unnecessary contact or travel and self-isolate if showing symptoms of infection.
• Coronavirus information from the NHS
• The UK Government’s advice on coronavirus
• WHO information page
• Science Media Centre Factsheet
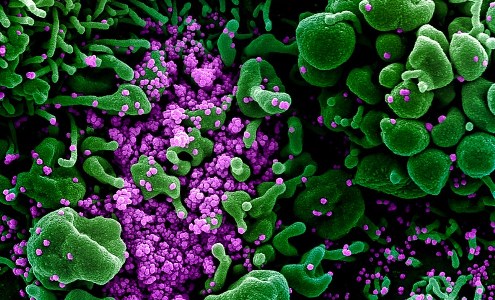 Colourised scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
Colourised scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

