In silico, in vivo

September 11th 2023
A remarkable new technique could pave the way for tiny electronic circuits to be ‘printed’ directly into the tissue of the body, write Dr John G Hardy, Dr Alexandre Benedetto and Dr John B Appleby
Electronics are ubiquitous in our everyday lives and now assist us in most of our daily activities. Electronics also underpin healthcare systems worldwide and telemedicine is a growing healthcare sector, with the potential to facilitate exciting innovations for our health – including wearable and implantable devices capable of sensing/stimulating physiological functions, or driving or enhancing prosthetics¹.
Clinically approved devices capable of electrical stimulation of the body – for example, bionic ears and eyes, cardiac pacemakers and electrodes for deep brain stimulation – currently rely on electrodes designed for long-term implantation. These electrodes are used in devices for a huge range of conditions, including neurological disorders, sensory deficits, spinal cord injury and strokes, and to control prosthetics2,3,4.
Typically manufactured from inorganic materials, they are not only chemically but also mechanically very different from the soft tissues in our bodies. Such mechanical mismatches promote tissue inflammation and, over time, the encapsulation of electrodes in fibrous scar tissue that impedes their functions2,3,4.
The surgical procedures used to implant such devices are complex and invasive, further fuelling
a growing market for new bioelectronics and ways to implant them.
Smart Approaches
Electronic components are typically manufactured using a layer-by-layer approach that makes their production scalable for medical and technical applications. Additive manufacturing – the industrial name for 3D printing – is fast becoming a method of choice for material and device production, notably due to the ease of sharing process blueprints.
New additive manufacturing technologies are being developed that could revolutionise the production of electronics5. As an example, we and our collaborators have taken advantage of a light-sensitive ‘smart ink’ material, which hardens when exposed to a specific colour of light to make micron-sized 3D structures.
Our microscopy setup uses an ultra-fast laser beam of infrared light that can activate the ink with high precision. This triggers the polymerisation of the smart ink to form defined structures. Once polymerised, the smart ink becomes conductive and can be used to build electronic circuits. It is essentially 3D-printing electronics5,6.
We began with proof-of-concept studies to print an electrical circuit within a flexible and transparent plastic matrix made from a common type of silicone called polydimethylsiloxane (PDMS). With Damian Cummings at University College London, we then used the electrical contact points printed on the plastic to stimulate neurones within a mouse brain tissue slice that was kept alive in vitro, evoking neuronal responses that were like those seen in vivo.
After tweaking the smart ink to maximise its biocompatibility, we aimed to directly print micro-structures in vivo on live microscopic roundworms (Caenorhabditis elegans). We then had to ensure the light used to polymerise the ink did not burn the worms. Immobilising wriggling worms during the 3D printing procedure was another challenge, which we addressed by anaesthetising the worms and trapping them in PDMS microgrooves moulded from 12in vinyl records – the spacing of their grooves is perfect for adult worms.
Because the roundworms are transparent, it was possible to focus the laser beam within them and print structures in their gut and on their ‘skin’. As their transparency makes them more vulnerable to heat, light and desiccation than most human tissues, printing on these animals and keeping them alive was a significant step towards making the technology less risky for human/veterinary applications.
The next steps in the journey towards the application of direct bioprinting of electronics in vivo will involve optimisation of a variety of parameters. These include, but are not limited to, ink formulations (biocompatibility, interactions with biological tissues, polymerisation kinetics/thermodynamics and physicochemical properties of the polymers produced); photon focus and intensity to enhance print fidelity; exploration of the scope of tissues in which it is possible to print; and the delivery of functional prototypes to potential end users.
There is significant excitement around the development of novel additive manufacturing techniques for the production of electronic devices, discussed further in an excellent review from Divakaran et al7. The rapidly expanding field of bioprinting is also covered in reviews from Bejoy8, Fang9, Noroozi10 and their colleagues. Our work, which is the first to combine bioprinting and bioelectronics, complements work on printing nonconductive polymer structures in vivo using near-infrared (NIR) light sources, which has shown that 3D hydrogels can be bioprinted in live mice11 and that personalised ear-like tissue constructs can be bioprinted, also in live mice12.
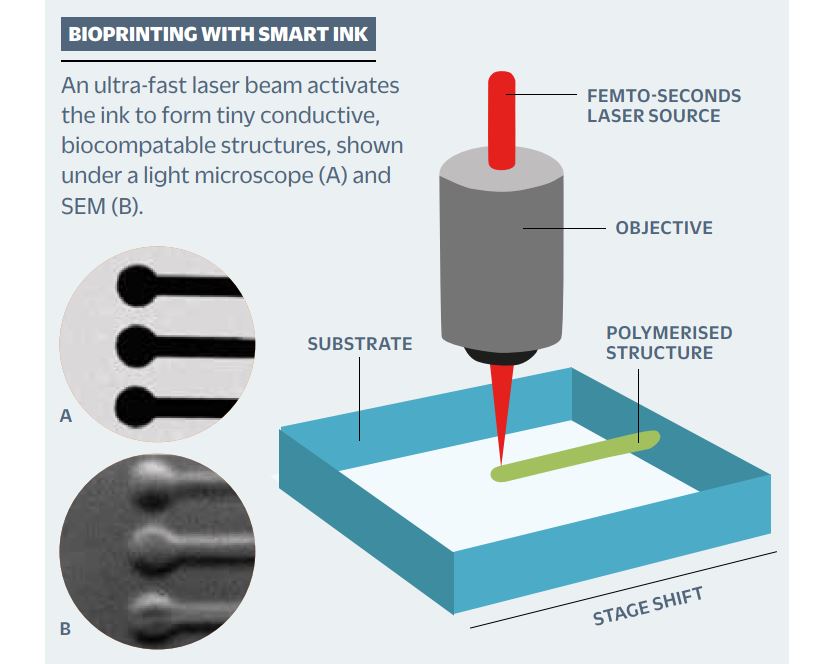 A diagram showing the basic concept of 3D bioprinting with laser-activated ink (right), plus examples of microscopic bioprinted structures (left).
A diagram showing the basic concept of 3D bioprinting with laser-activated ink (right), plus examples of microscopic bioprinted structures (left). For example, wearable biocompatible printed micro-electronics will contribute to the development of human-computer interfaces. These may be developed with the intention of improving treatment of neurological conditions, but such technology could also be used by a bad actor in such a way that negatively affects the privacy and autonomy of the individuals using them.
One way to negotiate future dual-use dilemmas responsibly is to take steps to try to identify them in advance, and subsequently have researchers work with regulators to creatively design ethical safeguards that can be engineered into and alongside the development of the technologies.
For example, one safeguarding procedure may involve keeping key aspects of research knowledge secure that would otherwise enable the harmful use of such research. Insofar as possible, researchers must endeavour to keep engaging in horizon-scanning in relation to future stages of this research to anticipate and tackle ethical dilemmas.
Analogical Leap
The potential technical advantages of electronics produced via this technique could include more accurate targeting – i.e. devices stimulating or recording from fewer cells; minimising the adverse effects of, say, tissue damage or immunological or reaction/inflammation; and increased specificity, efficiency and effectiveness, with the potential of more simultaneous sites for stimulation or recording with optimised signal-to-noise ratios.
With increasing and ageing populations worldwide, further advancement of this technology could lead to improved and better tolerated electrodes, novel neuroprosthetics (for example, for traumatic brain injury), new approaches to monitoring or treating neuromuscular disorders, and novel approaches to neuromodulation for pain. The direct printing of conducting polymer structures directly on/in living organisms could also enable real-time repairs of implanted bioelectronic devices. In short, the technique could have significant economic, environmental, health and societal impacts.
Those in academia, government or industry with an interest in collaborating with us are encouraged to make contact.
Dr John G Hardy is a senior lecturer in materials chemistry in the Department of Chemistry and Materials Science at Lancaster University. He uses expertise in chemistry, engineering and pharmacy to develop novel materials for bioelectronic applications.
Dr Alexandre Benedetto is a senior lecturer in integrated physiology in the Division of Biomedical and Life Sciences, C4AR & LIRA, at Lancaster University. He uses Caenorhabditis elegans as a model system to study the microbiota-gut-brain axis in the contexts of ageing, stress and death.
Dr John B Appleby is a lecturer in medical ethics working at Lancaster Medical School and is an associate director at the Centre for Global Eco-Innovation at Lancaster University. He studies the ethical and regulatory issues raised by medicine and innovative biotechnologies.
References
1) Tulkoff, C. & Caswell, G. Design for Excellence in Electronics Manufacturing (Wiley, New York, 2021).
2) Pethig, R. & Smith, S. Introductory Bioelectronics: For Engineers and Physical Scientists (John Wiley & Sons Ltd, Chichester, 2012).
3) Katz, E. Implantable Bioelectronics: Devices, Materials, and Applications (Wiley-VCH, 2014).
4) Stavrinidou, E. & Proctor, C. M. Introduction to Bioelectronics: Materials, Devices, and Applications (AIP Publishing, 2023).
5) Baldock, S. J. et al. Creating 3D objects with integrated electronics via multiphoton fabrication in vitro and in vivo. Adv. Mater. Technol. 8(11), 2201274 (2023).
6) Hardy, J. G. Photoinitiating polymerisable composition. PCT/GB2017/052235, EP3493859A1, GB2562443A, GB2562443B, US20190175796A1, WO2018025026A1.
7) Divakaran, N. et al. Comprehensive review on various additive manufacturing techniques and its implementation in electronic devices. J. Manuf. Syst. 62, 477–502 (2022).
8) Bejoy, A. M. et al. An insight on advances and applications of 3D bioprinting: A review. Bioprinting. 24, e00176 (2021).
9) Fang, Y. et al. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Additive Manufacturing Frontiers 1(1), 100011 (2022).
10) Noroozi, R. et al. 3D and 4D bioprinting technologies: A game changer for the biomedical sector? Ann. Biomed. Eng. 51, 1683–1712 (2023).
11) Urciuolo, A. et al. Nat. Biomed. Eng. 4, 901–915 (2020).
12) Chen, Y. et al. Noninvasive in vivo 3D bioprinting. Sci. Adv. 6(23), aba7406 (2020).


