Working (a synchrotron) from home

Above: Professor Sir David Stuart. Image courtesy of Diamond Light Source.
During the pandemic researchers have been operating the Diamond Light Source synchrotron remotely, running crucial COVID-related experiments on its beamlines from the comfort of their own homes. Post-pandemic, remote and automated experimentation is likely to become the norm, says structural biologist and director of life science at Diamond Professor Sir David Stuart.
December 15th 2021
Diamond Light Source is a particle accelerator in Oxfordshire designed to produce intense beams of X-rays, Infra Red and UV light to aid scientific research. During the COVID-19 pandemic, the facility has remained operational, prioritising work on pandemic-related research, for example work to understand the interactions between the viral spike protein and human antibodies, or the screening of potentially useful therapeutic compounds.
Sir David Stuart is Life Sciences Director at Diamond, joint Head of Structural Biology at the University of Oxford, and a Medical Research Council Professor of Structural Biology at the Wellcome Trust Centre for Human Genetics. He is best known for his work elucidating the structure of viruses, and was recently knighted for his contribution to structural biology and his work on the COVID-19 pandemic.
He tells Tom Ireland about some of the insights the Diamond beamlines have been able to provide – and how researchers can now operate technology at the facility remotely and automate entire experiments.
Hi David. Can you tell us a bit about what the synchotron has been able to do, in terms of providing insights into the structure of SARS-CoV-2 and the macromolecules associated with it?
Diamond provides both cryo-electron microscopes and X-ray capability. The cryo-electron microscopes are not significantly different to the electron microscopes in top labs around the world, but I think the difference is that we are organised to run the microscopes as intensively as possible. They're very efficiently run, and we’ve been able to keep them working throughout the pandemic so far. There's been a lot of electron microscopy work looking at structures of spike protein, spike-antibody complexes and some work on the viral polymerase. We're able to allow people in, perhaps from smaller labs, who wouldn't have access to the equipment at their institutions.
It's quite different on the x-ray crystallography side. X-ray crystallography really is done almost entirely nowadays at synchrotrons. For any difficult project or any project where you need to move quickly, you need access to a synchrotron, which can give you really high resolution atomic-detail structures of the proteins that you're interested in and at high throughput.
Where synchrotrons may have really moved forward is introducing automation into the process, and Diamond I think it's fair to say has been one of the leading light sources in doing this. Our robotics and automation give you the opportunity of doing things remotely. This is something that Diamond introduced a few years ago now and has been gradually doing more and more of, having the users send samples by courier. Then the user remotely drives the experiments and collects the data to solve the structure.
 Professor Sir Dave Stuart, Director of Life Sciences at Diamond Light Source and Joint Head of Structural Biology at University of Oxford. Image courtesy of Diamond Light Source.
Professor Sir Dave Stuart, Director of Life Sciences at Diamond Light Source and Joint Head of Structural Biology at University of Oxford. Image courtesy of Diamond Light Source. So researchers can actually operate the beamline from anywhere around the world?
Yes, you have a little bit of software called NoMachine, which gives you a window onto the machines at Diamond, and it's as if you were there – you get the control system for the beamline. It's actually taken a little while to set up because you have to make sure you know who's in control of the system – but it works beautifully.
Now, especially during the lockdown, we're developing the next thing, which is a complete automation of the system which we call unattended data collection. You fill out all the information on a web form about what's in each of the little pins that you send into Diamond. That information is then used by us to design the experiment that will be done, then essentially the software queues up all of the experiments and executes them. It can run very efficiently through the night.
Normally users operate the beamline on the more difficult experiments in the day, and then the automated stuff kicks in overnight. But, because the algorithms have got better, the quality of the data collected automatically now means that in many cases it's very hard for most users to do better. So our lab now, where we can, uses unattended data collection. You just send these things off, keep an eye on the database that you put the original information into, and then the structures start coming back at some point.
Absolutely fascinating – we’ve all being working from home a lot but operating a particle accelerator from across the country is taking it to another level.
If you go back a few years with cryo-electron microscopes they were very much hands on – people spending hours in a dark room. There has been a real shift and the software there has gradually got better too. Before COVID, all the users always came to do the experiment with cryo-electron microscopes. Now, we have no users on site and again we use the virtual machine software to let the users drive the experiment remotely.
Do you think this level of automation – i.e. researchers not even needing to come to the facility – will continue post COVID?
Well, we won't go back to what we were at the beginning. But there is always going to be some experiments where there's a real benefit for the user to being on site. The advantage of the automation and the robotics is that the user can concentrate on the real problem, which is getting the good biological sample.
If you can increase the throughput, then you can think about doing different sorts of experiments. You can look at samples that were too risky to justify bothering with, or you can look at a large number of crystals and pick the best one with the interesting results. That allows you then to dissect out different conformational states because the spike is actually not a rigid molecule – there's quite a lot of flexibility and that's really biologically important. For example, for the ACE2 receptor to bind, the receptor binding domain has to point upwards on the spike to reveal the binding site.
The variant viruses now, both the Kent, and the Brazilian, and the South African, not only have mutations in the spike, they also have mutations in the N terminal domain which is way away, right on the outside. The best explanation for that at the moment is that this modulates the presentation of the binding site. That’s a case where if you collect a lot of data from the electron microscope you can then start to tease apart different conformational states and get important biological insight.
Can this sort of structural biology help us get a head start in understanding which mutations might be important or worrying for transmission or vaccine efficacy? Or do you really just have to wait until case numbers of a particular variant rise to understand which mutations are advantageous?
I think understanding how and where the ACE2 receptor binds and what mutations do around that site is a way that you can perhaps pick up worrying things more rapidly.
The question really is can you design therapies that can get around the potential problem of a variant virus escaping immune responses? You might, for instance, put additional sugars on to hide some of the sites and focus the immune response elsewhere. But also if you can stop the receptor binding domains going up, to be recognised by ACE2, that would be a good neutralisation mechanism for an antiviral – the exact conformation of the spike protein wouldn’t be so important.
Or, if you could convert the prefusion state of the spike to a post fusion state, then that would kill the virus, its machinery of getting in would be destroyed. So I think there may be alternatives that get around this problem of variants. I think long term, what we really need are good antivirals.
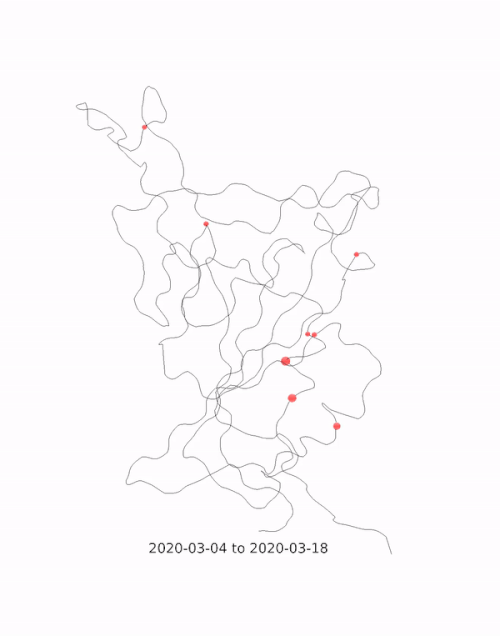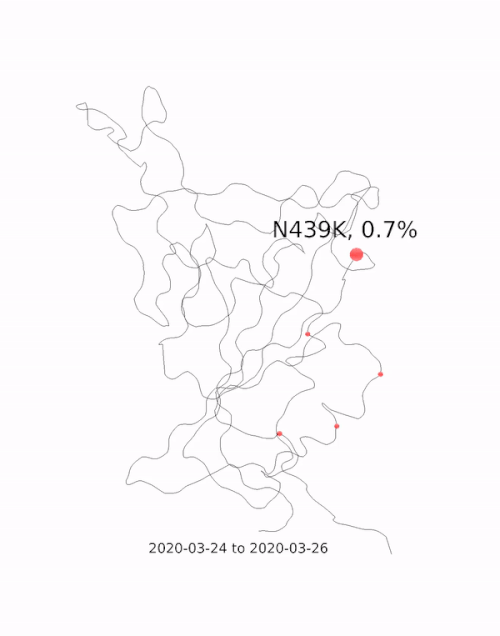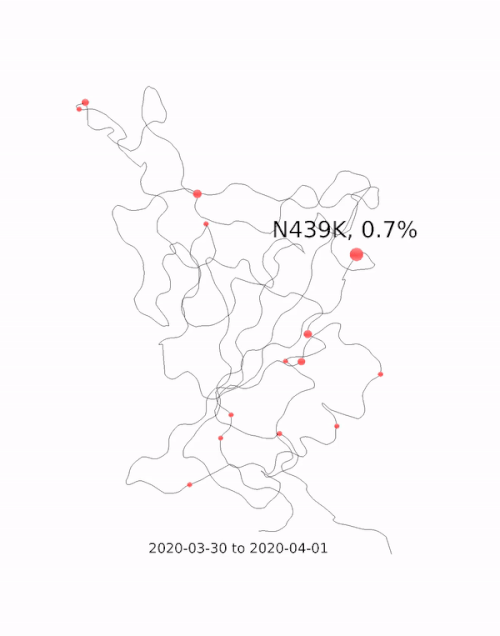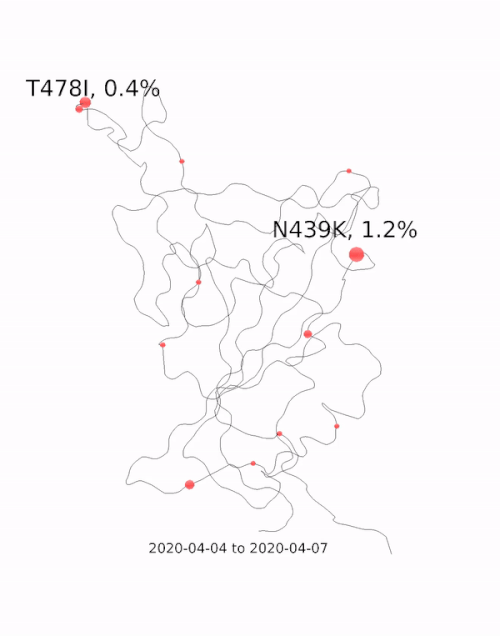 This animation by Helen Gill, a postdoc at the University of Oxford and Diamond Light Source, shows the relative prevalence of mutations and the location of the amino acid change on the binding domain of the SARS-CoV-2 spike protein. The large red circle appearing at the end of 2020 is the 501y mutation, a key feature of the so-called 'Kent variant'. Video courtesy of COG-UK.
This animation by Helen Gill, a postdoc at the University of Oxford and Diamond Light Source, shows the relative prevalence of mutations and the location of the amino acid change on the binding domain of the SARS-CoV-2 spike protein. The large red circle appearing at the end of 2020 is the 501y mutation, a key feature of the so-called 'Kent variant'. Video courtesy of COG-UK.Can you tell me a bit about the drug repurposing project being done at Diamond?
There's a drug discovery company in Oxford called Exscientia which negotiated access to a library of about 12,000 approved drugs and drugs that have made it through some sort of trial in people. They got access to that and could do the initial screening against a number of targets: the main protease, the papaine-like protease, and also a portion of the spike the receptor binding domain, because we thought it would be interesting to see if it was possible to get compounds that would stop receptor attachment to the spike.
So they did initial screening and as compounds came up as potentially interesting they were then fed back to Diamond for the structural analysis.
On the basis of that work, there's a small number of compounds have been identified. They were then taken to testing in cells, to check the effectiveness at neutralising the virus or knocking down virus replication in cells. Then the next stage will be to put them into animals. I think they still need some more work before they could go into human efficacy trials, but hopefully they're not far off.
Has Diamond prioritised COVID-related work this year and essentially shut down to other research?
COVID is given a priority, although we are doing other things. We are currently running round the clock four days a week instead of our usual six days (the seventh day is usually devoted to machine physics). Very early on we decided that we would set up a rapid access call that didn't go through the normal peer review routes, but was just reviewed by two or three people internally within Diamond just to get things moving quickly. There was a period when if we hadn't done that we couldn't have justified keeping operating - all other work in universities was shutting down.
Although a lot of people repurposed their research towards COVID-19, there is still far less research going on than normal. That meant we were actually able to give more access, with fewer constraints, than we would do normally when there's a greater demand for the facility.
Professor Sir David Stuart is Life Science Director at Diamond Light Source, Medical Research Council Professor of Structural Biology at the Wellcome Trust Centre for Human Genetics, joint head of Structural Biology at the University of Oxford, and a Fellow of Hertford College, Oxford. He was recently knighted for his contribution to structural biology and his work on the COVID-19 pandemic.
This interview was conducted at the end of January 2021.
Find out more about the Diamond Light Source at www.diamond.ac.uk


